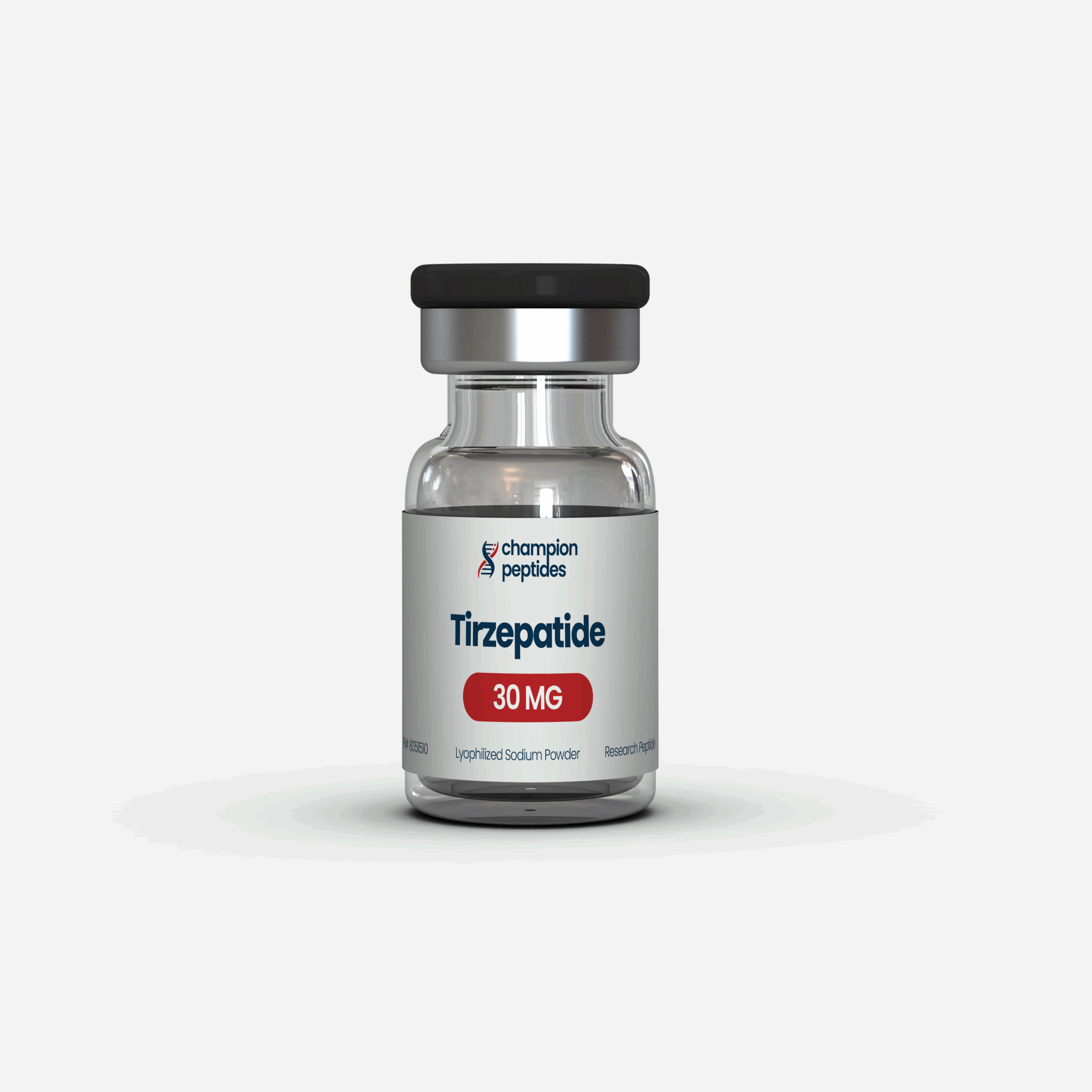
Tirzepatide 30mg + Kit
$130.00
Tirzepatide 30mg + Kit: A research-grade package containing 30mg of Tirzepatide, a dual GLP-1 and GIP receptor agonist peptide, and a reconstitution kit for preparing the peptide for use. Tirzepatide promotes significant weight loss, improves glycemic control, and enhances insulin sensitivity by suppressing appetite and regulating glucose metabolism, with potential benefits for metabolic health. The reconstitution kit typically includes bacteriostatic water (e.g., 3ml vial), insulin syringes, a mixing syringe, and alcohol prep pads to facilitate sterile preparation and administration of the lyophilized peptide for research purposes.
Tirzepatide + Kit
What is Tirzepatide + Kit?
Tirzepatide + Kit is a research-grade package containing Tirzepatide, a dual glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist, and a reconstitution kit for preparing the peptide for use. Tirzepatide promotes significant weight loss, improves glycemic control, and enhances metabolic health by suppressing appetite, slowing gastric emptying, and improving insulin sensitivity. The reconstitution kit includes supplies to ensure sterile preparation of the lyophilized peptide for research purposes.
How It Works (Mechanism of Action)
Tirzepatide activates both GLP-1 and GIP receptors, amplifying their synergistic effects on metabolism:
- GLP-1 Receptor: Enhances glucose-dependent insulin secretion, suppresses glucagon release, slows gastric emptying, and reduces appetite, promoting satiety and lower caloric intake.
- GIP Receptor: Augments insulin release, improves fat metabolism, and may enhance energy expenditure, contributing to weight loss and metabolic efficiency.
This dual agonism results in greater weight reduction and glycemic control compared to single GLP-1 agonists, targeting multiple pathways to regulate glucose, appetite, and fat metabolism.
Potential Benefits & Use Cases
- Significant Weight Loss: Clinical trials report up to 20.9% body weight reduction at 72 weeks (15 mg dose) in obese or overweight individuals, with 36.2% achieving ≥25% weight loss.
- Glycemic Control: Reduces HbA1c by up to 2.34% in type 2 diabetes, with 90% of patients achieving HbA1c <7% (SURPASS-5 trial).
- Metabolic Health: Enhances insulin sensitivity, reduces visceral fat, and improves lipid profiles, potentially slowing kidney disease progression.
- Cardiovascular Benefits: Lowers cardiovascular risk by 38% in heart failure with preserved ejection fraction and obesity; improves cholesterol and blood pressure.
- Research Applications: Studied for obesity, type 2 diabetes, non-alcoholic fatty liver disease, chronic kidney disease, and obstructive sleep apnea.
Typical Dosage & Administration
Administration: Subcutaneous injection, typically once weekly, in the abdomen, thigh, or upper arm.
Reconstitution Process:
- Kit Contents: Typically includes a 3ml vial of bacteriostatic water, 8 insulin syringes (0.5-1ml, 27-31 gauge), 1 mixing syringe (e.g., 3cc, 20-25 gauge), and 8 alcohol prep pads.
- Steps: Disinfect vials with alcohol pads, draw 1-3 ml bacteriostatic water with the mixing syringe, slowly inject along the vial wall to minimize foam, gently swirl (do not shake), and store at 2-8°C. Use within 30 days; avoid freeze-thaw cycles.
- Concentration Example: Reconstituting 30mg with 3ml water yields 10mg/ml (1000mcg = 0.1ml). For a 5mg dose, draw 0.5ml (50 units on a 1ml, 100-unit syringe).
Dosage:
- Starting Dose: 2.5 mg weekly for 4 weeks to minimize gastrointestinal side effects.
- Titration: Increase by 2.5 mg every 4 weeks to a maximum of 5, 10, or 15 mg weekly, based on research protocol and tolerability.
- Protocol Example: 2.5 mg (weeks 1-4), 5 mg (weeks 5-8), 7.5 mg (weeks 9-12), up to 15 mg (week 17+). A 30mg vial supports ~12 doses at 2.5 mg or ~2 doses at 15 mg. Consult a professional for precise regimens.
Possible Side Effects
- Gastrointestinal Issues: Nausea (26%), diarrhea (15%), vomiting (13%), or constipation, most common during dose escalation, typically mild and transient.
- Injection Site Reactions: Redness, swelling, or irritation, minimized with proper sterile technique.
- Hypoglycemia: Rare (0.4 events/year), primarily with sulfonylureas or insulin; monitor in metabolic studies.
- Other: Fatigue, dizziness, or increased lipase; rare risks of pancreatitis or hypersensitivity reactions.
Side effects are generally manageable with dose titration and monitoring.
Other Relevant Details
- FDA Approval: Approved as Mounjaro (2022) for type 2 diabetes and Zepbound (2023) for obesity/overweight with comorbidities and obstructive sleep apnea.
- Storage: Lyophilized vials stable at 2-8°C; reconstituted solutions at 2-8°C for 30 days or -20°C with carrier protein. Avoid freeze-thaw cycles.
- Kit Utility: Simplifies preparation for multiple doses (e.g., 30mg vial yields ~6 doses at 5 mg or ~2 doses at 15 mg).
- Research Context: Superior to semaglutide in weight loss (SURMOUNT-5), with ongoing trials for kidney and cardiovascular outcomes.
- Not for Human Consumption: Intended for laboratory or in-vitro research only outside FDA-approved indications, per supplier guidelines.
[](https://en.wikipedia.org/wiki/Tirzepatide)
[](https://www.purerxpeptides.com/product-page/tirzepatide-kits)
[](https://www.drugs.com/tirzepatide.html)